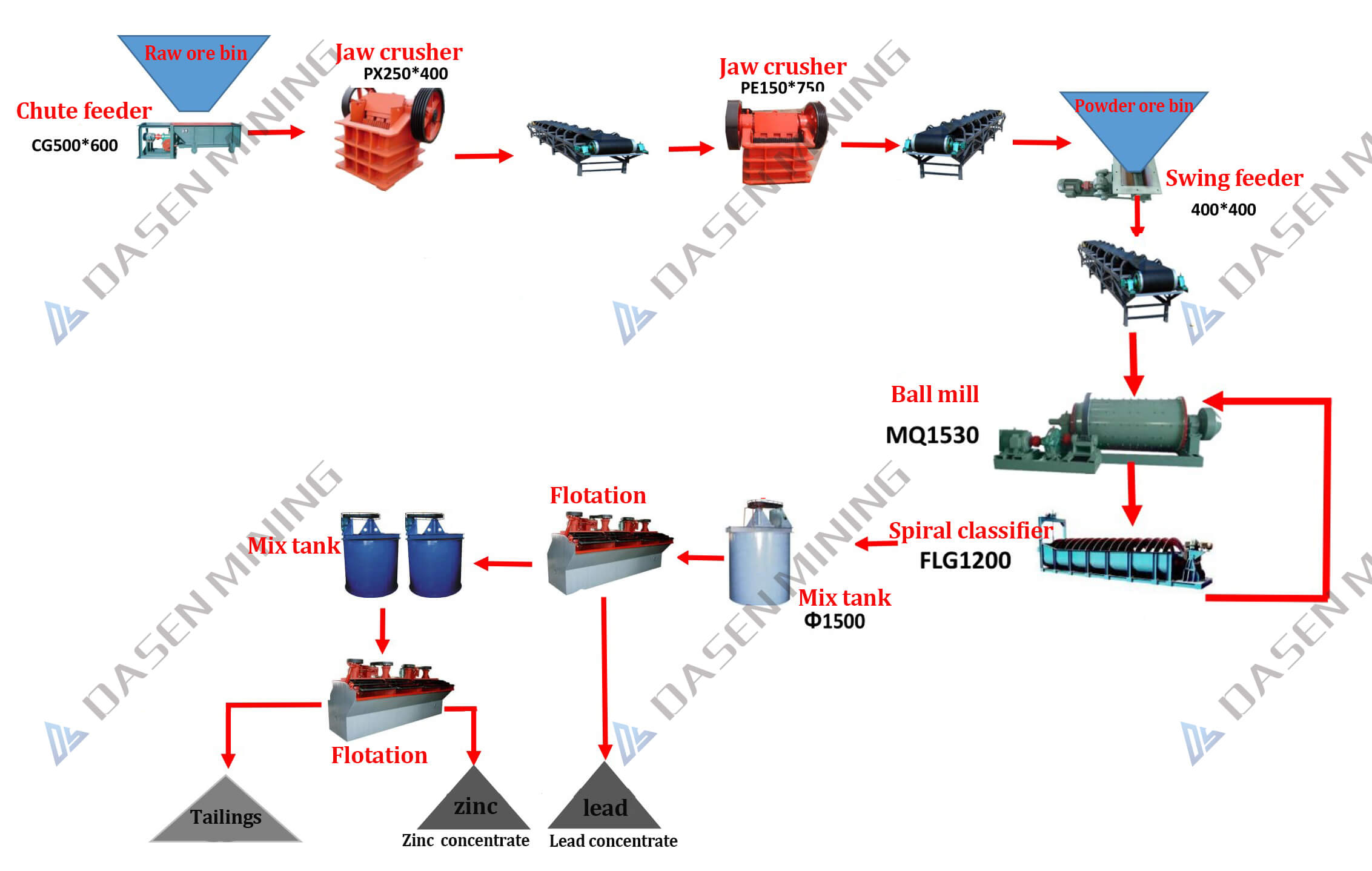
From lead-zinc ore, how can silver mining process?
Understanding the Silver in Your Ore
Before embarking on a heap leaching operation, it’s crucial to understand the nature of the silver in your lead-zinc ore. Is it readily soluble silver like angular silver ore or natural silver, or is it more complex, like dark red or light red silver ore? The answer will significantly impact your leaching strategy.
The Role of Cyanide Leaching
Cyanide leaching is a widely used technique for extracting silver from ores. However, it’s essential to consider the potential challenges posed by other minerals, particularly zinc. Zinc oxide minerals, while easily soluble in cyanide, can consume significant amounts of cyanide and generate zinc cyanide complexes that can hinder silver dissolution.
Balancing Act: Lead and Cyanide
Lead, while often considered a nuisance in gold and silver extraction, can play a beneficial role in certain scenarios. It can help precipitate sulfide ions, promoting silver dissolution. However, excessive lead can negatively impact the leaching process.
Optimizing Heap Leaching for Silver Recovery
To maximize silver recovery from your lead-zinc ore, consider the following strategies:
High Cyanide Concentrations: Employing higher concentrations of cyanide solution can enhance silver dissolution.
Optimal Porosity and Permeability: Ensure your heap has good porosity and permeability to facilitate oxygen diffusion, a critical factor in silver oxidation and dissolution.
Careful pH Control: Maintain a suitable pH level to optimize cyanide leaching efficiency.
Regular Monitoring and Control: Implement rigorous monitoring and control systems to track key parameters like cyanide concentration, pH, and oxygen levels.
Regular Monitoring and Control: Implement rigorous monitoring and control systems to track key parameters like cyanide concentration, pH, and oxygen levels.
You can send us a private letter if you don’t know anything about the silver mining process:
Whatsapp:+86 133 1927 7356
Email:[email protected]